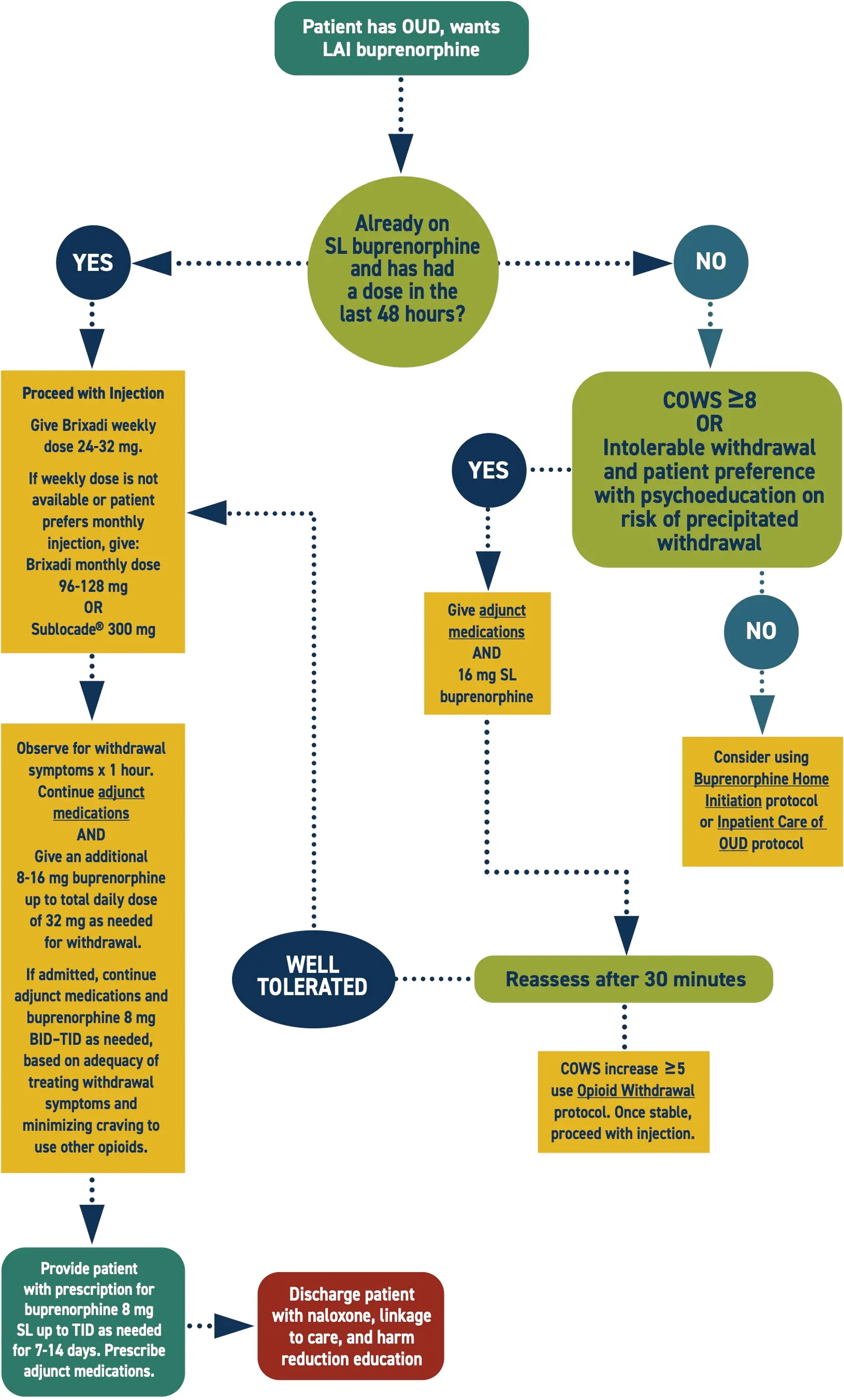
Considerations
This protocol suggests using the Brixadi® weekly dose to initiate treatment due to lower risk of opioid withdrawal. Other LAI buprenorphine formulations are also apppropriate.
Engage in shared decision making with the patient prior to initiating treatment. Inform of the risk of precipitated withdrawal, especially with COWS <8 and/or concurrent stimulant intoxication.
Potential complicating factors include:
- Allergy or sensitivity
- Severe respiratory compromise
- Chronic use of long acting opioids (e.g., methadone or Oxycontin®)
Consider expert consultation, but prioritize treating symptoms.
It can take several months for the injection to achieve full clinical effect. Prescriptions for SL buprenorphine and adjunct medications facilitate retention in care.
Consider screening for HIV, HCV, STIs, and mental health comorbidities.
Additional Clinical Guidance
- Opioid use disorder (OUD) is a treatable health condition. It is best treated with methadone or buprenorphine.
- Buprenorphine and methadone are life-saving medications for OUD that reduce the risk of all-cause mortality and overdose death by over 50%.
- Recovery often requires multiple treatment attempts. A repeat encounter is not a treatment failure, but an opportunity to reinitiate potentially life-saving medication.
- If patients continue to use other opioids while on methadone or buprenorphine, a higher dose may be needed to manage their symptoms. Therapeutic dosing should be guided by adequate management of withdrawal and cravings.
- Opioid withdrawal is excruciating. Without swift and adequate intervention, patients may self-direct discharge and be at risk for overdose.
- Patients with OUD are highly stigmatized. Stigma prevents people from seeking care and worsens health outcomes. Providers should challenge biases to provide compassionate, evidence-based care.
Assessment
- Assess the Clinical Opiate Withdrawal Scale (COWS) score before administering buprenorphine. For this initiation approach, only administer buprenorphine if the patient has signs of withdrawal.
- Reassess vitals, COWS, and subjective experience of withdrawal within 30 minutes of buprenorphine administration.
Labs
- Drug testing is not necessary to initiate treatment for OUD.
- If drug testing is performed for clinical reasons, obtain informed consent.
Pharmacotherapy
- Buprenorphine is a partial opioid agonist that helps to minimize withdrawal and lessen opioid cravings and use.
- Long-acting injectable buprenorphine (LAIB) is ideal for patients with OUD who have difficulty taking daily medication or do not want to take daily medication.
- LAIB provides extended protection against overdose as it gradually tapers. If a dose is missed, the slow taper reduces the severity of withdrawal symptoms.
- LAIB ensures the patient receives the medication consistently and doses are not skipped.
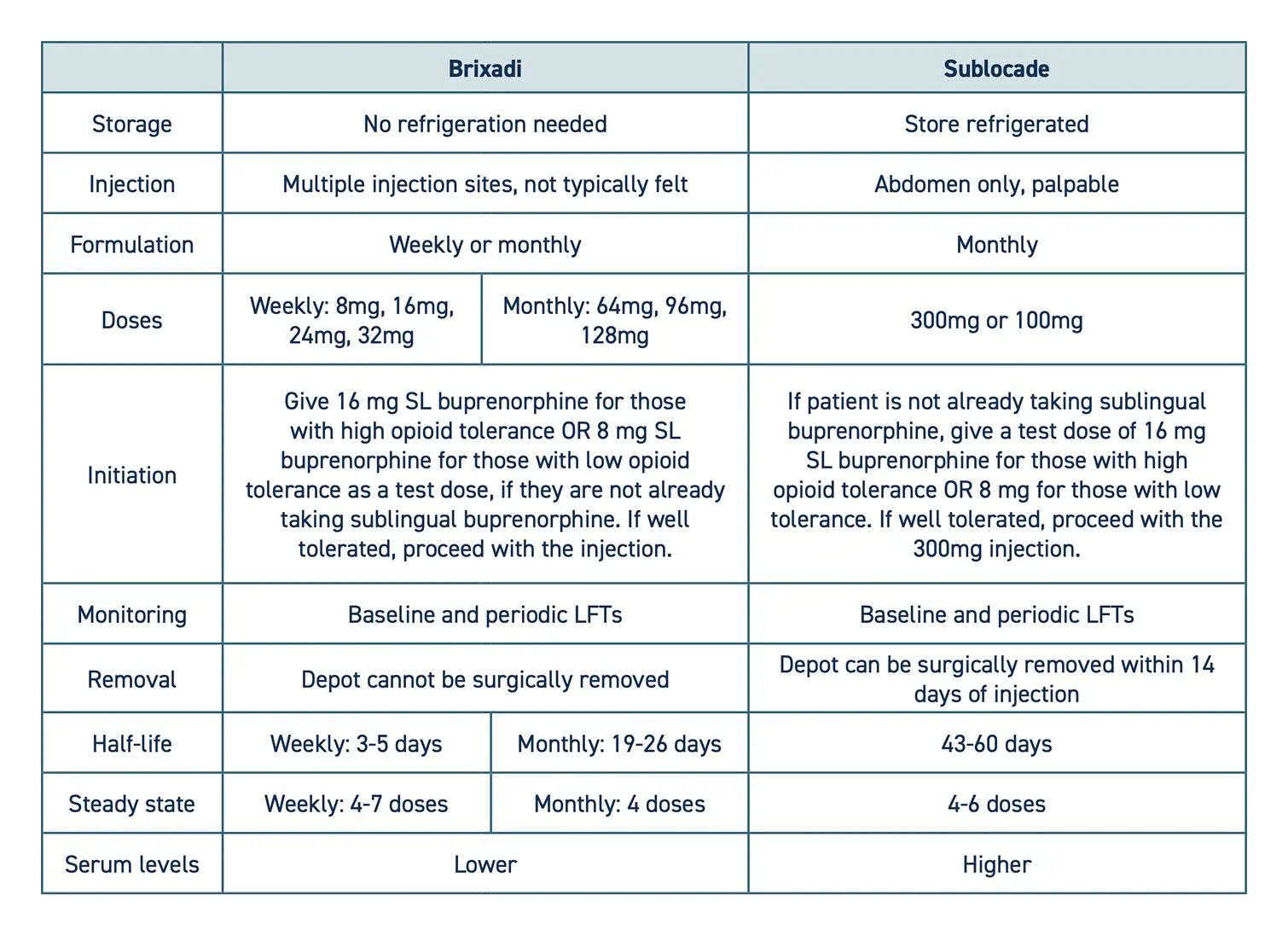
- This protocol suggests using Brixadi (weekly) to initiate treatment because of a lower risk of withdrawal symptoms; however, any LAI formulation is appropriate and recommended if your setting does not have Brixadi (weekly), or the patient prefers a longer-acting formulation.
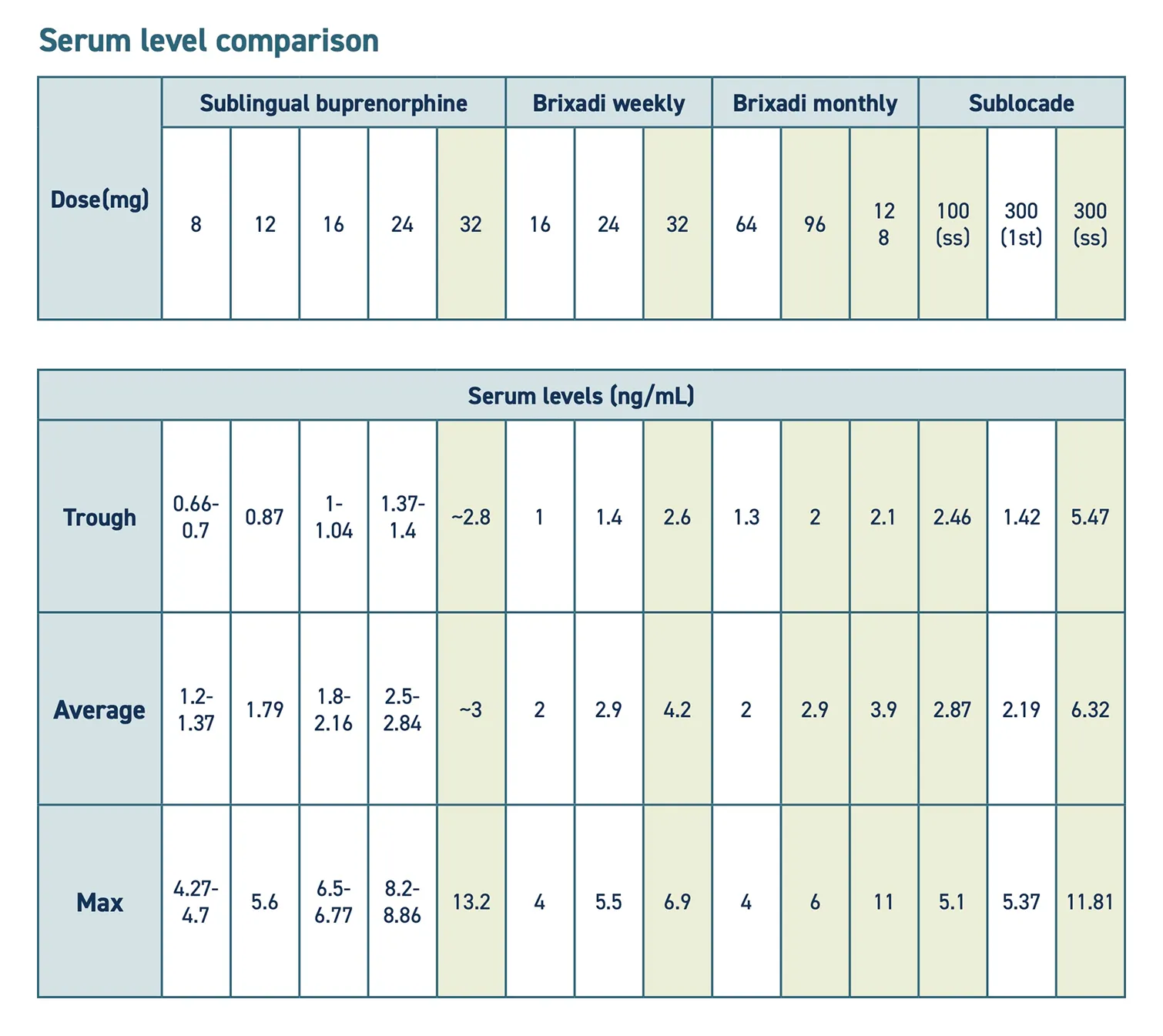
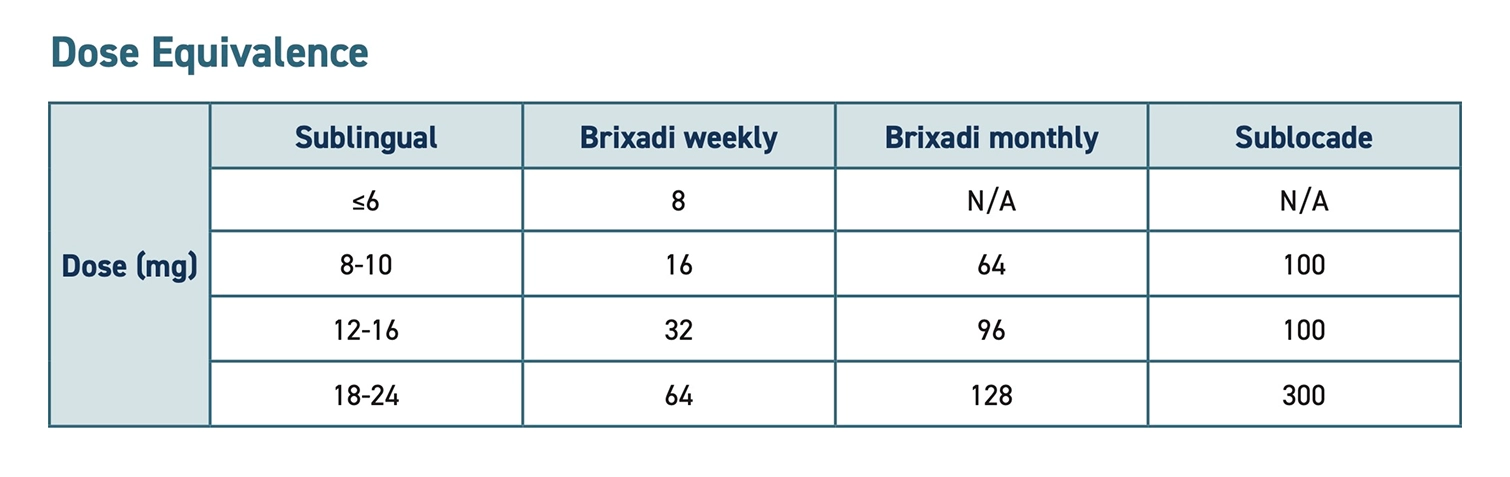
- The following is a comparison of the two available formulations:
Supplemental medications
- It may take several days for the injection to achieve clinical effect. Additional medications should be provided to ease withdrawal symptoms.
- Sublingual buprenorphine should be provided to all patients starting LAIB to manage withdrawal symptoms while adjusting to the medication.
- Some patients will also need sublingual buprenorphine to manage cravings as the time for their next injection approaches.
- Provide prescriptions for appropriate adjunct medications for withdrawal symptoms.
- Some patients may need to transition from Brixadi to Sublocade to adequately treat OUD symptoms, as it provides a higher serum level.
Polysubstance use
- Active stimulant intoxication can falsely elevate the COWS score.
- Buprenorphine administration may unmask symptoms of stimulant intoxication.
- Polysubstance use is never a contraindication for initiating buprenorphine or methadone.
Patient safety
- Administer by subcutaneous injection only. Serious harm or death may result if administered intravenously. Long-acting injectable buprenorphine forms a solid mass when it comes into contact with body fluids.
Administration
- LAI buprenorphine formulations are administered via subcutaneous injection. Never inject intravenously, intramuscularly, or intradermally.
- Pre-administration preparation:
- If using Sublocade, allow it to reach room temperature. Brixadi does not require refrigeration.
- Put patient in supine position.
Identify an injection site in the subcutaneous tissue of the buttock, thigh, abdomen, or upper arm.
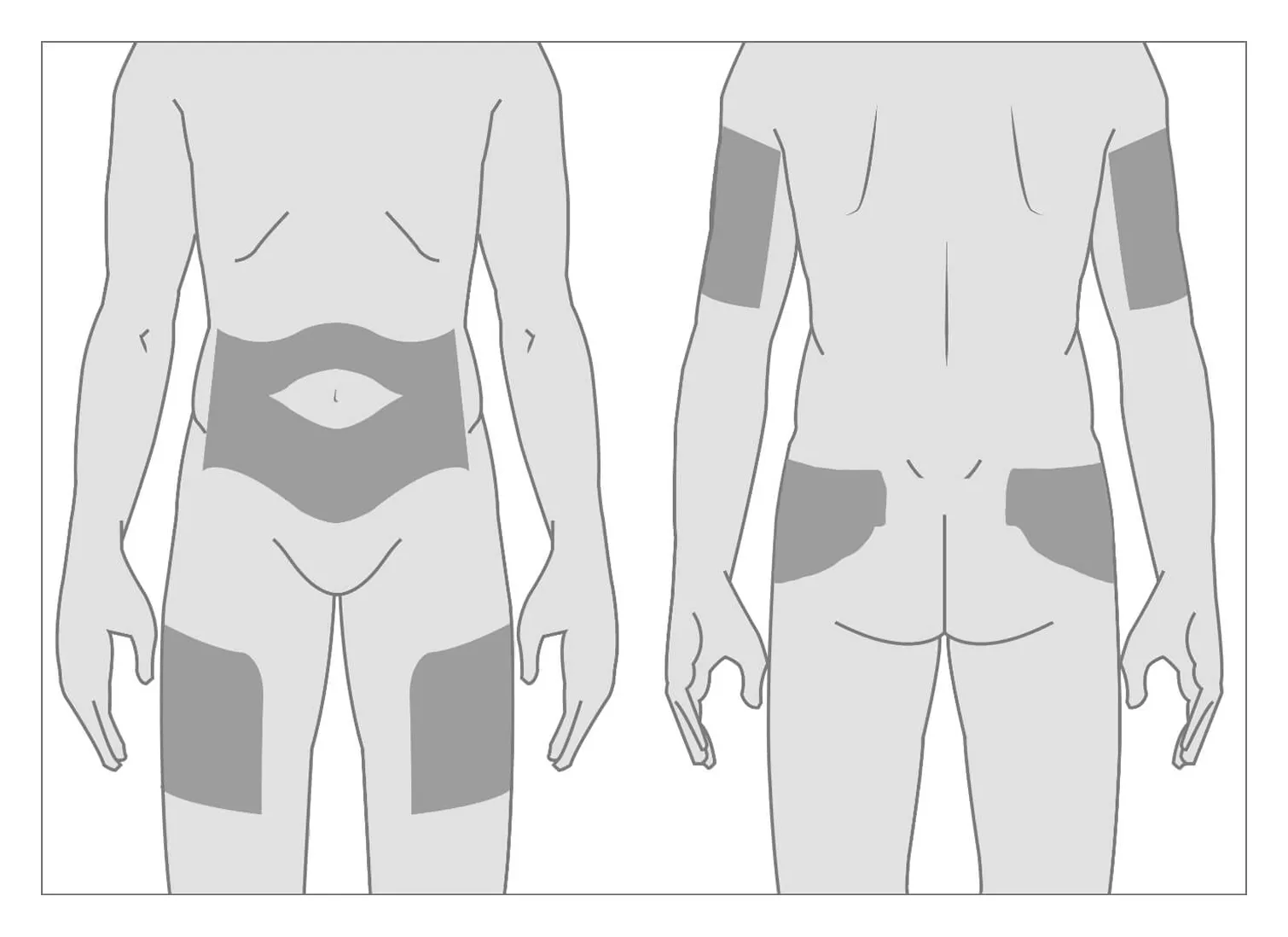
Image source: https://www.brixadihcp.com/dosing-and-administration/
- Inspect the injection site for rashes, open sores, scars, or skin irregularities. If these are present, select a different site.
- Pain management:
- Patients may experience a burning sensation during injection. This is more commonly reported with Sublocade than Brixadi. Ensuring the patient has a good experience with the injection will help them engage in ongoing care for follow-up injections.
- Minimize discomfort with one or more of the following strategies:
- Prep the area with an ice pack directly on skin for 20 minutes
- Lidocaine (intradermal/subcutaneous or EMLA cream)
- Mindfulness techniques
- Administration:
- Administer subcutaneously at 45-90° angle, depending on amount of adipose tissue. Inject slowly.
- The medication will form a solid mass that slowly absorbs over time. Sublocade creates a palpable mass, whereas Brixadi may not be palpable.
- Document the site of injection. Sites must be rotated to prevent irritation.
- Advocate for all patients to receive additional sublingual buprenorphine and adjunct medications for withdrawal symptoms and administer as prescribed.
Discharge planning
- Help the patient schedule a follow-up appointment. Hospitals enrolled in ScalaNW can call the 24/7 appointment scheduling line and receive a confirmed date, time, and location for MOUD follow up appointment during the 10-minute phone call.
- For hospitals not enrolled in ScalaNW, the Washington Recovery Helpline MOUD Locator (online or at 1-866-789-1511) is a useful resource for finding OUD treatment in Washington.
- Provide the patient with a buprenorphine prescription to last until their scheduled outpatient appointment. When possible, prescribe 3 additional days beyond the appointment date to allow for barriers or rescheduling. If no appointment is scheduled, provide at least 7-14 days of medication to give the patient time to secure an appointment.
- Patients can call the Washington Telebuprenorphine Hotline (206-289-0287) if they run out of medication prior to their follow up appointment.
- Provide the patient with discharge instructions that include the time of the last dose and when to take the next dose. Ensure the patient understands the importance of taking buprenorphine at around the same time every day.
- Many patients need adjunct medications to control withdrawal symptoms until they stabilize on buprenorphine. If needed, provide prescriptions for adjunct withdrawal management medications to cover at least 7 days.
- In Washington, emergency departments are required to dispense naloxone to patients with OUD or others who are at risk of opioid overdose, in compliance with SB5195. Ensure patient is discharged with naloxone in hand.
- When possible, connect patients with supports such as social workers, care navigators, or peers to improve patient experience and strengthen linkage to care.
Patient education
Educate the patient about:
- Buprenorphine administration:
- Must be administered under the tongue for proper absorption.
- It can take 5-10 minutes for the medication to fully absorb. Avoid eating, drinking, smoking, or talking during this time.
- Drinking water prior to administration can help it dissolve faster.
- To prevent oral decay, rinse mouth with water 30 minutes after administration.
- The risks of combining sedatives with buprenorphine, which can cause respiratory depression.
- The importance of avoiding driving or operating machinery until accustomed to the medication. Provide work notes if needed.
- Overdose prevention strategies. Ensure the patient and their support system understand when and how to use naloxone.
- The risks of change in use patterns, which can alter tolerance and increase risk of opioid overdose.
- The need to establish follow-up care before the next injection is due.
Discharge Instructions
Information about buprenorphine
- Buprenorphine is a safe and effective medication used to treat opioid use disorder (OUD).
- Buprenorphine helps people with OUD break the cycle of use and withdrawal, feel more stable, and focus on other parts of their lives so they can recover.
- When used as prescribed, buprenorphine significantly lowers the risk of opioid overdose if you take other opioids.
- There are two kinds of buprenorphine. Buprenorphine-naloxone also contains naloxone. Naloxone is not absorbed when the medication is taken as directed (under the tongue). If the medication is injected, however, the naloxone will be absorbed and can cause severe opioid withdrawal.
- There is no limit to how long a person can take buprenorphine. It is recommended that most people be on the medication long-term.
- Side effects may occur and are typically mild and improve over time. These can include constipation, sweating, headache, dizziness, trouble sleeping, nausea, and sleepiness. If these occur, notify your health care provider, nurse, or pharmacist.
- Over time, your body will adjust to buprenorphine. If you stop taking it suddenly, you will feel withdrawal symptoms within a few days.
- A common side effect of buprenorphine and other opioid medications is constipation. To prevent constipation: stay hydrated, eat plenty of fiber, and start taking an over-the-counter stool softener. If you do not have a bowel movement in over 24-48 hours, try an over-the-counter laxative. Talk to the pharmacist to help choose the best one for you.
How to take oral buprenorphine
- You will receive buprenorphine in either film or tablet form.
- Do not swallow the medicine. It will not work if swallowed.
- Place the medication under your tongue and allow it to fully dissolve. This can take 5-15 minutes.
- Drinking water before taking buprenorphine may help it dissolve faster.
- Do not drink water while the medication is under your tongue.
- Do not eat, drink, talk, or smoke while the medication is dissolving.
- After the medication has dissolved, do not smoke, eat, or drink for at least 15 minutes.
- To prevent tooth decay, rinse your mouth with water 30 minutes after taking the medication.
CAUTION
- Continue taking your regular buprenorphine dose, even if you feel better. Stopping may cause withdrawal and cravings. Your risk of overdose will be much higher if you use opioids without being on this medication.
- Do not start taking or increase your use of other sedative medications like benzodiazepines. The combination of buprenorphine and other sedatives could make you so sleepy that you may stop breathing.
- Do not drink more than your usual amount of alcohol while starting buprenorphine.
- Do not drive when you first start buprenorphine because it may slow your reaction time. Wait until you know how it affects you.
- Remember that changes in opioid use patterns can alter your tolerance and increase your risk of opioid overdose. If you use other opioids, take steps to lower the risk—carry naloxone, never use alone, or call the Never Use Alone lifeline (877-696-1996).
Buprenorphine dose and time taken
________mg @___:____
Overdose prevention
- Using drugs is risky. If you use drugs, lower your risk of dying from an opioid overdose with the following strategies:
- Naloxone
- Today you received naloxone or a prescription for naloxone. This is an opioid overdose reversal medication. It is safe to use on anyone you suspect is experiencing an opioid overdose.
- Visit stopoverdose.org or talk to your provider, nurse, or pharmacist to learn more.
- Try not to use alone
- If you are not with other people, connect with a confidential service like neverusealone.com. This peer-led service will send someone to help if you stop responding during a chat or phone call.
- Start low & go slow
- You can't know the full contents or strength of drugs. If you use, start with a very small amount to see how it affects you.
- Be extra cautious if you have low tolerance (for example, after not using for awhile). If you decide to use more, slowly increase in small amounts.
- Watch and wait before next person uses
- If you’re with a group of people, take turns to see how the drug is affecting people. Someone needs to be able to ask for help.
- Avoid mixing drugs
- Mixing drugs increases your risk. If you use multiple drugs, try to use one at a time and use less of each.
- Know the signs of opioid overdose and how to respond.
- If someone is unresponsive or has unusual or no breathing, call 911 and give them naloxone and rescue breaths.
- Always have naloxone
- Tell others you have it, where it is, and when to use it.
- Treatment with methadone or buprenorphine
- These medications, if taken as directed, lower the risk of death by over 50%.
- If you need help finding a treatment provider, call the Washington Recovery Helpline at 866-789-1511 or go to warecoveryhelpline.org.
References
- Andorn AC, Haight BR, Shinde S, et al. Treating Opioid Use Disorder With a Monthly Subcutaneous Buprenorphine Depot Injection: 12-Month Safety, Tolerability, and Efficacy Analysis. J Clin Psychopharmacol. 2020;40(3):231-239. doi:10.1097/JCP.0000000000001195
- Braeburn. Dosing Options. BrixadiHCP.com. Published 2025. Accessed August 16, 2025. https://www.brixadihcp.com/dosing-and-administration/
- Brixadi. Prescribing information. Braeburn Pharmaceuticals; 2024. Accessed August 16, 2025. https://www.brixadi.com/pdfs/brixadi-prescribing-information.pdf
- D'Onofrio G, Hawk KF, Herring AA, et al. The design and conduct of a randomized clinical trial comparing emergency department initiation of sublingual versus a 7-day extended-release injection formulation of buprenorphine for opioid use disorder: Project ED Innovation. Contemp Clin Trials. 2021;104:106359. doi:10.1016/j.cct.2021.106359
- D'Onofrio G, Herring AA, Perrone J, et al. Extended-Release 7-Day Injectable Buprenorphine for Patients With Minimal to Mild Opioid Withdrawal. JAMA Netw Open. 2024;7(7):e2420702. doi:10.1001/jamanetworkopen.2024.20702
- D'Onofrio G, Perrone J, Hawk KF, et al. Early emergency department experience with 7-day extended-release injectable buprenorphine for opioid use disorder. Acad Emerg Med. 2023;30(12):1264-1271. doi:10.1111/acem.14782
- Frost M, Bailey GL, Lintzeris N, et al. Long-term safety of a weekly and monthly subcutaneous buprenorphine depot (CAM2038) in the treatment of adult out-patients with opioid use disorder. Addiction. 2019;114(8):1416-1426. doi:10.1111/add.14636
- Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778-790. doi:10.1016/S0140-6736(18)32259-1
- Johnson B, Monwell B, Capusan AJ. Long-acting injectable depot buprenorphine from a harm reduction perspective in patients with ongoing substance use and multiple psychiatric comorbidities: a qualitative interview study. Harm Reduct J. 2024;21(1):68. doi:10.1186/s12954-024-00984-1
- Jones AK, Ngaimisi E, Gopalakrishnan M, Young MA, Laffont CM. Population Pharmacokinetics of a Monthly Buprenorphine Depot Injection for the Treatment of Opioid Use Disorder: A Combined Analysis of Phase II and Phase III Trials. Clin Pharmacokinet. 2021;60(4):527-540. doi:10.1007/s40262-020-00957-0
- Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. J Addict Med. 2015;9(5):358-367. doi:10.1097/ADM.0000000000000166
- LeSaint KT, Kendric KJ, Logan AA. Successful administration of extended-release buprenorphine in the emergency department. Am J Emerg Med. 2024;84:189.e1-189.e3. doi:10.1016/j.ajem.2024.07.046
- Lintzeris N, Dunlop AJ, Haber PS, et al. Patient-Reported Outcomes of Treatment of Opioid Dependence With Weekly and Monthly Subcutaneous Depot vs Daily Sublingual Buprenorphine: A Randomized Clinical Trial. JAMA Netw Open. 2021;4(5):e219041. doi:10.1001/jamanetworkopen.2021.9041
- Lofwall MR, Walsh SL, Nunes EV, et al. Weekly and Monthly Subcutaneous Buprenorphine Depot Formulations vs Daily Sublingual Buprenorphine With Naloxone for Treatment of Opioid Use Disorder: A Randomized Clinical Trial. JAMA Intern Med. 2018;178(6):764-773. doi:10.1001/jamainternmed.2018.1052
- Marion-Bellemare L, Srivastava A, Samson J, et al. Initiation of extended-release buprenorphine in emergency department patients: A retrospective cohort study. Am J Emerg Med. 2025;94:71-75. doi:10.1016/j.ajem.2025.04.011
- Matheson C, Foster R, Schofield J, Browne T. Long-acting depot buprenorphine in people who are homeless: Views and experiences. J Subst Abuse Treat. 2022;139:108781. doi:10.1016/j.jsat.2022.108781
- Neale J, Tompkins CNE, Strang J. Prolonged-release opioid agonist therapy: qualitative study exploring patients' views of 1-week, 1-month, and 6-month buprenorphine formulations. Harm Reduct J. 2019;16(1):25. doi:10.1186/s12954-019-0296-4
- Rosenwohl-Mack S, Suen LW, Logan AA, Peterson D, Snyder HR. Outpatient Initiation of 7-Day Injectable Buprenorphine: A Direct-to-Inject Case Series. Subst Use Addict J. 2025;0(0). doi:10.1177/29767342251330412
- Sublocade. Prescribing information. Indivior Inc; 2024. Accessed August 16, 2025. https://www.sublocade.com/Content/pdf/prescribing-information.pdf


 I’M A CLINICIAN
I’M A CLINICIAN I’M A PATIENT
I’M A PATIENT