Considerations
Fentanyl use and fentanyl withdrawal in pregnancy is associated with a high risk of parental overdose death and preterm labor, in addition to other pregnancy and delivery related complications. Transition to MOUD greatly reduces these risks, is safer and more effective than withdrawal management (“detox”), and is recommended by the American College of Obstetricians and Gynecologists (ACOG).
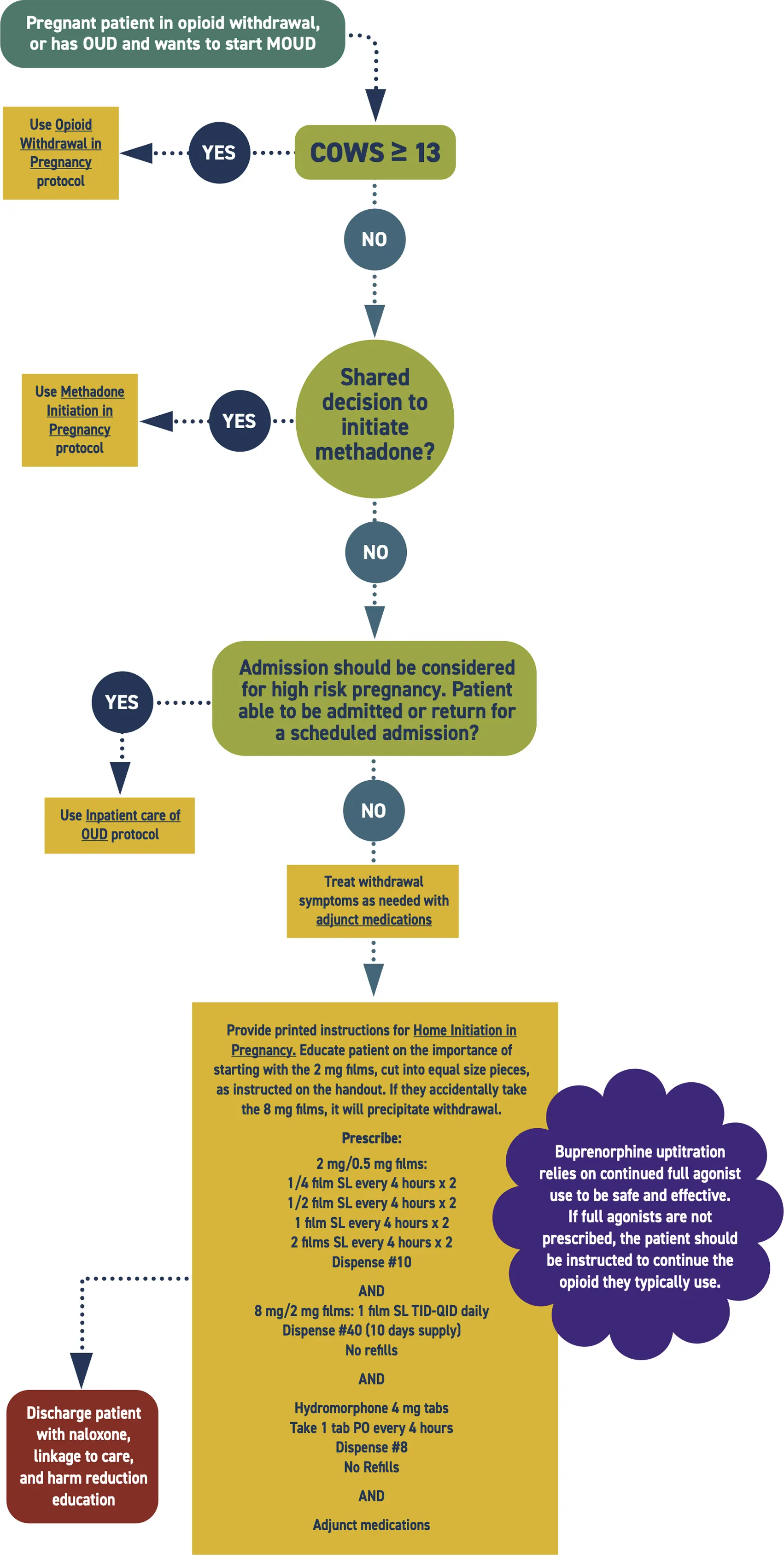
Admission should be considered to stabilize the pregnancy and support the transition to MOUD. Medicaid pays for medically necessary admissions.
The physiological stress of pain and withdrawal is experienced by both the parent and fetus. Stabilization and prevention of withdrawal benefits both members of the dyad. Using hydromorphone concurrently with the uptitration of buprenorphine is safe, minimizes the risk of harm to the pregnancy, and treats pain and hyperalgesia associated with withdrawal. Titrate buprenorphine to therapeutic levels prior to stopping hydromorphone.
Buprenorphine films are easier than tabs for this rapid uptitration schedule.
Potential complicating factors include:
- Severe respiratory compromise
- Concurrent sedative use
- Allergy or sensitivity
- Chronic use of long acting opioids (e.g., methadone or Oxycontin®)
Consider expert consultation, but prioritize treating symptoms.
Consider screening for HIV, HCV, STIs, and mental health comorbidities. Link to ongoing care as needed.
Patient Handouts
Additional Clinical Guidance
- Opioid use disorder (OUD) is a treatable health condition. It is best treated with methadone or buprenorphine.
- Buprenorphine and methadone are life-saving medications for OUD that reduce the risk of all-cause mortality and overdose death by over 50%.
- Recovery often requires multiple treatment attempts. A repeat encounter is not a treatment failure, but an opportunity to reinitiate potentially life-saving medication.
- If patients continue to use other opioids while on methadone or buprenorphine, a higher dose may be needed to manage their symptoms. Therapeutic dosing should be guided by adequate management of withdrawal and cravings.
- Opioid withdrawal is excruciating. Without swift and adequate intervention, patients may self-direct discharge and be at risk for overdose, pregnancy or delivery complications, harm to the fetus/newborn, and disruptions to the family system.
- The primary goal is to provide evidence-based, patient-centered care to perinatal patients with OUD, ensuring the safety and well-being of both patient and fetus/newborn. Medications for opioid use disorder (MOUD) enable stability, facilitate bonding, and support parental and infant health.
- Pregnant and parenting patients with OUD are highly stigmatized. Stigma prevents people from seeking care and worsens health outcomes. Providers should challenge biases to provide compassionate, evidence-based care.
Assessment
- Consider screening for sexually transmitted infections, including HIV, hepatitis C virus (HCV), syphilis, gonorrhea, and chlamydia. Consider linkage to PrEP as indicated.
- Consider screening for mental health comorbidities, including anxiety, depression, and post-traumatic stress disorder.
Labs
- Drug testing is not necessary to initiate treatment of OUD.
- If drug testing is performed for clinical reasons, obtain informed consent.
- In Washington State, drug use alone does not constitute a mandatory report to Child Protective Services (CPS).
Pharmacotherapy
- Buprenorphine is a partial opioid agonist that helps to minimize withdrawal and lessen opioid cravings and use.
- Both buprenorphine and combination buprenorphine/naloxone are safe during pregnancy and lactation.
- Higher doses of buprenorphine (at least 16-32 mg daily) are considered most effective.
- Higher doses do not increase the risk of neonatal opioid withdrawal and are safe during breastfeeding.
- Buprenorphine metabolizes faster later in pregnancy. Patients may require higher doses or more frequent dosing (up to every 6 hours) of sublingual buprenorphine to maintain therapeutic levels.
- Patients with OUD have increased opioid tolerance and may require higher doses of opioids for effective pain management, including during labor, delivery, and postpartum.
Pathophysiology
- Consider admission for buprenorphine initiation in pregnant patients. Admission allows for close monitoring of withdrawal symptoms and swift intervention to address precipitated withdrawal should it occur.
- Severe opioid withdrawal in pregnancy is extremely high risk. Withdrawal in pregnancy can lead to severe complications, including:
- Maternal autonomic instability and dehydration
- Fetal hypoxia and distress; in rare cases, fetal demise
- Uterine irritability triggering preterm labor
- Unregulated opioid use that may result in overdose
- Neonatal opioid withdrawal syndrome
- Fetal growth restriction
- Adverse epigenetic changes, potentially affecting long-term neurodevelopmental outcomes
- Perinatal patients with OUD are at increased risk for opioid overdose, particularly in the postpartum period, due to changes in opioid tolerance.
- Neonatal opioid withdrawal syndrome (NOWS), previously referred to as neonatal abstinence syndrome (NAS), is an expected and treatable potential outcome for neonates exposed to opioids in utero.
- The risk of NOWS should not discourage buprenorphine treatment. Higher doses do not increase the risk of neonatal opioid withdrawal.
Polysubstance use
- Active stimulant intoxication can falsely elevate the COWS score.
- Buprenorphine administration may unmask symptoms of stimulant intoxication.
- Polysubstance use is never a contraindication for initiating buprenorphine or methadone.
Discharge planning
- Help the patient schedule a follow-up appointment for OUD treatment. Hospitals enrolled in ScalaNW can call the 24/7 appointment scheduling line and receive a date, time, and location for medications for opioid use disorder (MOUD) follow up appointment during the 10-minute phone call.
- For hospitals not enrolled in ScalaNW, The Washington Recovery Helpline MOUD Locator (online or at 1-866-789-1511) is a useful resource for finding OUD treatment in Washington.
- Arrange follow-up appointment with an obstetric/postpartum provider. Assist the patient in scheduling a follow-up appointment, if possible. Provide referrals if needed.
- Provide the patient with a buprenorphine prescription to last until their scheduled outpatient appointment. When possible, prescribe 3 additional days beyond the appointment date to allow for barriers or rescheduling. If no appointment is scheduled, provide at least 7-14 days of medication to give the patient time to secure an appointment.
- Patients can call the Washington Telebuprenorphine Hotline (206-289-0287) if they run out of medication prior to their follow up appointment.
- Provide the patient with discharge instructions that include the time of the last dose and when to take the next dose. Ensure the patient understands the importance of taking buprenorphine at around the same time every day.
- Many patients need adjunct medications to control withdrawal symptoms until they stabilize on buprenorphine. If needed, provide prescriptions for adjunct withdrawal management medications to cover at least 7 days.
- In Washington, emergency departments are required to dispense naloxone to patients with OUD or others who are at risk of opioid overdose in compliance with SB5195. Ensure patient is discharged with naloxone in hand.
- When possible, connect patients with supports such as social workers, care navigators, or peers to improve patient experience and strengthen linkage to care.
Patient Education
Educate the patient about:
- The safety and effectiveness of buprenorphine during pregnancy and lactation. Emphasize the importance of continued buprenorphine treatment and encourage breastfeeding.
- The importance of starting/continuing buprenorphine and engaging in perinatal care to optimize maternal and neonatal outcomes.
- Buprenorphine administration
- Must be administered under the tongue for proper absorption.
- It can take 5-10 minutes for the medication to fully absorb. Avoid eating, drinking, smoking, or talking during this time.
- Drinking water prior to administration can help it dissolve faster.
- To prevent oral decay, rinse mouth with water 30 minutes after administration.
- The risks of combining sedatives with buprenorphine, which can cause respiratory depression.
- The importance of avoiding driving or operating machinery until accustomed to the medication. Provide work notes if needed.
- Overdose prevention strategies. Ensure the patient and their support system understand when and how to use naloxone.
- The risks of change in use patterns, which can alter tolerance and increase risk of opioid overdose.
Discharge Instructions
Information about buprenorphine
- Buprenorphine is a safe and effective medication used to treat opioid use disorder (OUD).
- Buprenorphine helps people with OUD break the cycle of use and withdrawal, feel more stable, and focus on other parts of their lives so they can recover.
- When used as prescribed, buprenorphine significantly lowers the risk of opioid overdose if you take other opioids.
- Buprenorphine-naloxone also contains naloxone. Naloxone is not absorbed when the medication is taken as directed (under the tongue). If the medication is injected, however, the naloxone will be absorbed and can cause severe opioid withdrawal.
- There is no limit to how long a person can take the buprenorphine. It is recommended that most people be on the medication long-term.
- Side effects may occur and are typically mild and improve over time. These can include constipation, sweating, headache, dizziness, trouble sleeping, nausea, and sleepiness. If these occur, notify your health care provider, nurse, or pharmacist.
- Over time, your body will adjust to the buprenorphine. If you stop taking it suddenly, you will feel withdrawal symptoms within a few days.
- A common side effect of methadone and other opioid medications is constipation. To prevent constipation: stay hydrated, eat plenty of fiber, and start taking an over-the-counter stool softener. If you do not have a bowel movement in over 24-48 hours, try an over-the-counter laxative. Talk to the pharmacist to help choose the best one for you.
Buprenorphine during pregnancy and breastfeeding
- Buprenorphine is safe to use while pregnant. At birth, the baby may have some withdrawal symptoms. These symptoms can be easily treated and do not cause lasting harm.
- Breastfeeding is safe and encouraged while taking buprenorphine.
- Breastfeeding is discouraged if you are actively using other drugs.
How to take the medication
- You will receive buprenorphine in either film or tablet form.
- Do not swallow the medicine. It will not work if swallowed.
- Place the medication under your tongue and allow it to fully dissolve. This can take 5-15 minutes.
- Drinking water before taking buprenorphine may help it dissolve faster.
- Do not drink water while the medication is under your tongue.
- Do not eat, drink, talk, or smoke while the medication is dissolving.
- After the medication has dissolved, do not smoke, eat, or drink for at least 15 minutes.
- To prevent tooth decay, rinse your mouth with water 30 minutes after taking the medication.
CAUTION
- Continue to take your regular buprenorphine dose even after you feel better even if you feel better. Stopping may cause withdrawal and cravings. Your risk of overdose will be much higher if you use opioids without being on this medication.
- Do not start taking or increase your use of other sedative medications like benzodiazepines. The combination of buprenorphine and other sedatives could make you so sleepy that you may stop breathing.
- Do not drink more than your usual amount of alcohol while starting buprenorphine.
- Do not drive when you first start buprenorphine because it may slow your reaction time. Wait until you know how it affects you.
- Remember that changes in opioid use patterns can alter tolerance and increase your risk of opioid overdose. If you use other opioids, take steps to lower the risk— carry naloxone, never use alone, or call the Never Use Alone lifeline (877-696-1996).
- Return to the emergency department if you experience any of the following: you are so sleepy that others are having a hard time waking you up, rashes, hives, wheezing, swelling of the face, difficulty breathing, problems with coordination, blurred vision, slurred speech, vomiting and can’t keep anything down, fever or severe pain, or you feel sicker.
Buprenorphine dose and time taken
________mg @___:____
Overdose Prevention
- Using drugs is risky. If you use, lower your risk of dying from an opioid overdose with the following strategies:
- Naloxone
- Today you received naloxone or a prescription for naloxone. This is an opioid overdose reversal medication. It is safe to use on anyone you suspect is experiencing an opioid overdose.
- Visit stopoverdose.org or talk to your provider, nurse, or pharmacist to learn more.
- Try not to use alone
- If you are not with other people, connect with a confidential service like neverusealone.com. This peer-led service will send someone to help if you stop responding during a chat or phone call.
- Start low & go slow
- You can't know the full contents or strength of drugs. If you use, start with a very small amount to see how it affects you.
- Be extra cautious if you have low tolerance (for example, after not using for awhile). If you decide to use more, slowly increase in small amounts.
- Watch and wait before next person uses
- If you’re with a group of people, take turns to see how the drug is affecting people. Someone needs to be able to ask for help, if it’s needed.
- Avoid mixing drugs
- Mixing drugs increases your risk. If you use multiple drugs, try to use one at a time and use less of each.
- Know the signs of opioid overdose and how to respond.
- If someone is unresponsive or has unusual or no breathing, call 911 and give them naloxone and rescue breaths.
- Always have naloxone
- Tell others you have it, where it is, and when to use it.
- Treatment with methadone or buprenorphine
- These medications, if taken as directed, lower the risk of death by over 50%.
- If you need help finding a treatment provider, call the Washington Recovery Helpline at 866-789-1511 or go to warecoveryhelpline.org.
References
- American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Opioids, Hatten BW, Cantrill SV, et al. Clinical Policy: Critical Issues Related to Opioids in Adult Patients Presenting to the Emergency Department. Ann Emerg Med. 2020;76(3):e13-e39. doi:10.1016/j.annemergmed.2020.06.049
- American College of Obstetricians and Gynecologists. Opioid use and opioid use disorder in pregnancy. ACOG Committee Opinion No. 711. Obstet Gynecol. 2017;130(2):e81-e94.
- Centers for Disease Control and Prevention. Opioid use disorder and pregnancy. Updated April 24, 2024. Accessed August 15, 2025. https://www.cdc.gov/opioid-use-during-pregnancy/treatment/
- Chambers LC, Hallowell BD, Zullo AR, et al. Buprenorphine dose and time to discontinuation among patients with opioid use disorder in the era of fentanyl. JAMA Netw Open. 2023;6(9):e2334540. doi:10.1001/jamanetworkopen.2023.34540
- Cohen SM, Straus E, Fiellin DA, et al. Hospital-Based Methadone and Buprenorphine Initiation Practices by Addiction Consult Services. JAMA Netw Open. 2025;8(8):e2526077. Published 2025 Aug 1. doi:10.1001/jamanetworkopen.2025.26077
- Crotty K, Freedman KI, Kampman KM. Executive Summary of the Focused Update of the ASAM National Practice Guideline for the Treatment of Opioid Use Disorder. J Addict Med. 2020;14(2):99-112. doi:10.1097/ADM.0000000000000635
- D'Onofrio G, O'Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474
- Englander H, Thakrar AP, Bagley SM, Rolley T, Dong K, Hyshka E. Caring for Hospitalized Adults With Opioid Use Disorder in the Era of Fentanyl: A Review. JAMA Intern Med. 2024;184(6):691-701. doi:10.1001/jamainternmed.2023.7282
- Grande LA, Cundiff D, Greenwald MK, Murray M, Wright TE, Martin SA. Evidence on buprenorphine dose limits: a review. J Addict Med. 2023;17(5):509-516. doi:10.1097/ADM.0000000000001189
- Herring AA, Perrone J, Nelson LS. Managing opioid withdrawal in the emergency department with buprenorphine. Ann Emerg Med. 2019;73(5):481-487. doi:10.1016/j.annemergmed.2018.11.032
- Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER) – approach, issues, and lessons learned. Addiction. 2012;107(suppl 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
- Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. J Addict Med. 2015;9(5):358-367. doi:10.1097/ADM.0000000000000166
- Khazanchi R, Wachman EM, Schiff DM, Modest A, Saia KA, Hsu HE. Mandatory child protective services reporting for substance-exposed newborns and peripartum outcomes: a difference-in-differences analysis. JAMA Pediatr. Published online May 6, 2024. doi:10.1001/jamapediatrics.2024.0903
- Link HM, Jones H, Miller L, Kaltenbach K, Seligman N. Buprenorphine-naloxone use in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2(3):100179. doi:10.1016/j.ajogmf.2020.100179
- Martin CE, Shadowen C, Thakkar B, et al. Buprenorphine dosing for the treatment of opioid use disorder through pregnancy and postpartum. Curr Treat Options Psychiatry. 2020;7(3):375-399. doi:10.1007/s40501-020-00221-z
- Min JE, Guerra-Alejos BC, Yan R, et al. Opioid coprescription through risk mitigation guidance and opioid agonist treatment receipt. JAMA Netw Open. 2024;7(5):e2411389. doi:10.1001/jamanetworkopen.2024.11389
- Patrick SW, Barfield WD, Poindexter BB, Committee on Fetus and Newborn, Committee on Substance Use and Prevention. Neonatal opioid withdrawal syndrome. Pediatrics. 2020;146(5):e2020029074. doi:10.1542/peds.2020-029074
- Reindel KL, DeAngelis MJ, Ferrara AS, et al. An exploratory study of Suboxone (buprenorphine/naloxone) film splitting: cutting methods, content uniformity, and stability. Int J Pharm Compd. 2019;23(3):258-263.
- Saia KA, Schiff D, Wachman EM, et al. Caring for pregnant women with opioid use disorder in the USA: expanding and improving treatment. Curr Obstet Gynecol Rep. 2016;5(3):257-263. doi:10.1007/s13669-016-0168-9
- Saxon AJ. Short-acting, full agonist opioids during initiation of opioid agonist treatment in the fentanyl era. JAMA Netw Open. 2024;7(5):e2411398. doi:10.1001/jamanetworkopen.2024.11398
- Young LW, Ounpraseuth ST, Merhar SL, et al; ACT NOW Collaborative. Eat, sleep, console approach or usual care for neonatal opioid withdrawal. N Engl J Med. 2023;388(25):2326-2337. doi:10.1056/NEJMoa2214470
- Washington State Department of Children, Youth, and Families. Plan of safe care: healthcare providers. DCYF website. Accessed August 14, 2025. https://www.dcyf.wa.gov/safety/plan-safe-care/Healthcare-Providers
- Zedler BK, Mann AL, Kim MM, et al. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: a systematic review and meta-analysis of safety in the mother, fetus and child. Addiction. 2016;111(12):2115-2128. doi:10.1111/add.13462


 I’M A CLINICIAN
I’M A CLINICIAN I’M A PATIENT
I’M A PATIENT