Considerations
Severe withdrawal in pregnancy is high risk. Withdrawal impacts placental function and increases the risk of stunted growth, preterm labor, fetal convulsions, and fetal death.
Pregnant patients with OUD should have their pain and withdrawal adequately managed regardless of, and prior to deciding, plans to continue treatment after discharge.
Opioid withdrawal during delivery is traumatic for the birthing patient and increases the risk and severity of neonatal opioid withdrawal syndrome (NOWS).
Monitor the patient and fetus closely while treating severe withdrawal.
Additional Clinical Guidance
- Opioid use disorder (OUD) is a treatable health condition. It is best treated with methadone or buprenorphine.
- Buprenorphine and methadone are life-saving medications for OUD that reduce the risk of all-cause mortality and overdose death by over 50%.
- Recovery often requires multiple treatment attempts. A repeat encounter is not a treatment failure, but an opportunity to reinitiate potentially life-saving medication.
- If patients continue to use other opioids while on methadone or buprenorphine, a higher dose may be needed to manage their symptoms. Therapeutic dosing should be guided by adequate management of withdrawal and cravings.
- Opioid withdrawal is excruciating. Without swift and adequate intervention, patients may self-direct discharge and be at risk for overdose, pregnancy or delivery complications, harm to the fetus/newborn, and disruptions to the family system.
- The primary goal is to provide evidence-based, patient-centered care to perinatal patients with OUD, ensuring the safety and well-being of both patient and fetus/newborn. Medications for opioid use disorder (MOUD) enable stability, facilitate bonding, and support parental and infant health.
- Pregnant and parenting patients with OUD are highly stigmatized. Stigma prevents people from seeking care and worsens health outcomes. Providers should challenge biases to provide compassionate, evidence-based care.
Assessment
- Consider screening for sexually transmitted infections, including HIV, hepatitis C virus, syphilis, gonorrhea, and chlamydia. Consider linkage to PrEP as indicated.
- Consider screening for mental health comorbidities, including anxiety, depression, and post-traumatic stress disorder.
Labs
- Drug testing is not necessary to initiate treatment of OUD.
- If drug testing is performed for clinical reasons, obtain informed consent.
- In Washington State, drug use alone does not constitute a mandatory report to Child Protective Services (CPS).
Pharmacotherapy
- Buprenorphine is a partial opioid agonist that helps minimize withdrawal and lessen opioid cravings.
- Buprenorphine has a respiratory depression ceiling and a higher affinity for opioid receptors than full agonist opioids. High receptor affinity can cause precipitated withdrawal, but it is also a protective factor against overdose death. Competitive occupation alongside partial activation reduces the risk of opioid overdose when other opioids are consumed while a patient is taking buprenorphine. Even a single dose provides protection in the high-risk 24-48-hour window after discharge.
- Doses of 16-32 mg daily are considered most effective for most patients. Doses should be adjusted based on symptoms—if they are having ongoing cravings to use, a dose increase should be considered. Higher doses do not increase the risk of neonatal opioid withdrawal and are safe during breastfeeding.
- Buprenorphine metabolizes faster later in pregnancy. Patients may require higher doses or more frequent dosing (up to every 6 hours) of sublingual buprenorphine to maintain therapeutic levels.
- Patients with OUD have increased opioid tolerance and may require higher doses of opioids for effective pain management, including during labor, delivery, and postpartum.
- Both buprenorphine and combination buprenorphine/naloxone are safe during pregnancy and lactation.
Pathophysiology
- Severe opioid withdrawal in pregnancy is extremely high risk. Withdrawal in pregnancy can lead to severe complications, including:
- Maternal autonomic instability and dehydration
- Fetal hypoxia and distress; in rare cases, fetal demise
- Uterine irritability triggering preterm labor
- Unregulated opioid use that may result in overdose
- Neonatal opioid withdrawal syndrome
- Fetal growth restriction
- Adverse epigenetic changes, potentially affecting long-term neurodevelopmental outcomes
- Perinatal patients with OUD are at increased risk for opioid overdose, particularly in the postpartum period, due to changes in opioid tolerance.
- Neonatal opioid withdrawal syndrome (NOWS), previously referred to as neonatal abstinence syndrome (NAS), is an expected and treatable potential outcome for neonates exposed to opioids in utero.
- The risk of NOWS should not discourage buprenorphine treatment. Higher doses do not increase the risk of neonatal opioid withdrawal.
Special populations
- Buprenorphine is FDA approved for patients aged 16 years and older and can be used off label in patients 13 years and older without parental consent in WA State. Pregnant patients under the age of 18 should have opiod withdrawal and opioid use disorder treated with medication.
Polysubstance use
- Active stimulant intoxication can falsely elevate the COWS score.
- Buprenorphine administration may unmask symptoms of stimulant intoxication.
- Polysubstance use is never a contraindication for initiating buprenorphine or methadone.
Administration
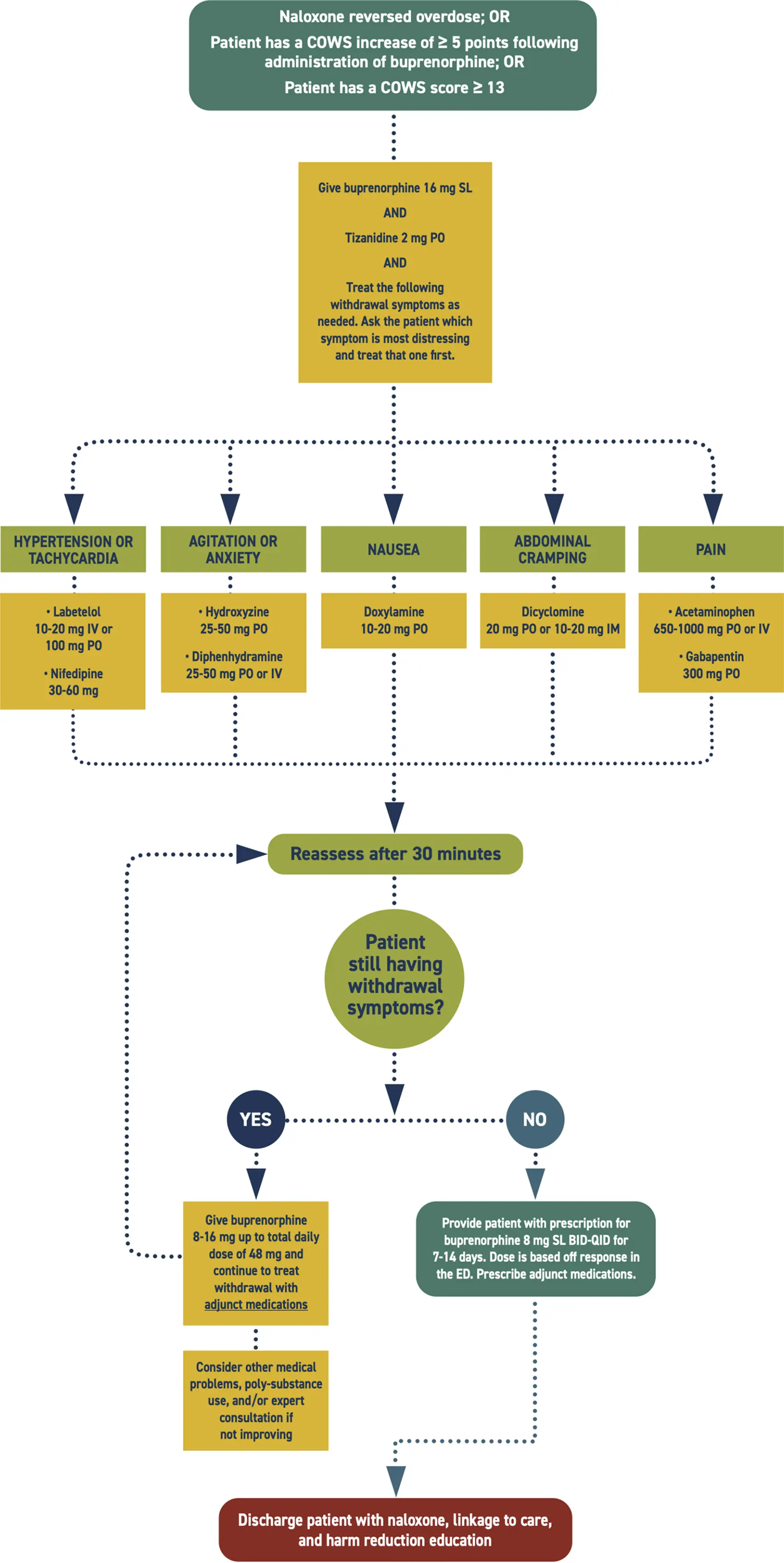
- Because buprenorphine is a partial agonist, if the first dose is administered too soon after recent full agonist opioid use in a patient with opioid dependence, there is a risk of precipitated withdrawal. This is a sudden worsening of withdrawal symptoms after buprenorphine administration. If this occurs, give additional doses of buprenorphine and adjunct medications to alleviate withdrawal symptoms.
- Administering buprenorphine:
- Buprenorphine comes in film and tablet forms, and both must be taken sublingually. The medication is poorly absorbed in the stomach. It must be taken correctly to receive the benefit.
- The patient should let the medication dissolve fully under their tongue. This typically takes 5-10 minutes, but sometimes longer. Drinking water prior to administration can help it dissolve faster. Many patients find the taste unpleasant. One way to know that the medication is dissolved and absorbed is when the taste subsides.
- Patients should not eat, drink, or talk while the medication is dissolving.
- To prevent oral decay, instruct the patient to rinse their mouth with water 30 minutes after administration.
- Administer adjunct medications, treating the symptoms the patient finds most distressing first.
Discharge planning
- Help the patient schedule a follow-up appointment. Hospitals enrolled in ScalaNW can call the 24/7 appointment scheduling line and receive a confirmed date, time, and location for MOUD follow up appointment during the 10-minute phone call.
- For hospitals not enrolled in ScalaNW, the Washington Recovery Helpline MOUD Locator (online or at 1-866-789-1511) is a useful resource for finding OUD treatment in Washington.
- Arrange follow-up appointment with an obstetric/postpartum provider. Assist the patient in scheduling a follow-up appointment, if possible. Provide referrals if needed.
- Provide the patient with a buprenorphine prescription to last until their scheduled outpatient appointment. When possible, prescribe 3 additional days beyond the appointment date to allow for barriers or rescheduling. If no appointment is scheduled, provide at least 7-14 days of medication to give the patient time to secure an appointment.
- Patients can call the Washington Telebuprenorphine Hotline (206-289-0287) if they run out of medication prior to their follow up appointment.
- Provide the patient with discharge instructions that include the time of the last dose and when to take the next dose. Ensure the patient understands the importance of taking buprenorphine at around the same time every day.
- Many patients need adjunct medications to control withdrawal symptoms until they stabilize on buprenorphine. If needed, provide prescriptions for adjunct withdrawal management medications to cover at least 7 days.
- When possible, connect patients with supports such as social workers, care navigators, or peers to improve patient experience and strengthen linkage to care.
Patient Education
Educate the patient about:
- Buprenorphine administration
- Must be administered under the tongue for proper absorption.
- It can take 5-10 minutes for the medication to fully absorb. Avoid eating, drinking, smoking, or talking during this time.
- Drinking water prior to administration can help it dissolve faster.
- To prevent oral decay, rinse mouth with water 30 minutes after administration.
- The risks of combining sedatives with buprenorphine, which can cause respiratory depression.
- The importance of avoiding driving or operating machinery until accustomed to the medication. Provide work notes if needed.
- Overdose prevention strategies. Ensure the patient and their support system understand when and how to use naloxone.
- The risks of change in use patterns, which can alter tolerance and increase risk of opioid overdose.
Discharge Instructions
Information about buprenorphine
- Buprenorphine is a safe and effective medication used to treat opioid use disorder (OUD).
- Buprenorphine helps people with OUD break the cycle of use and withdrawal, feel more stable, and focus on other parts of their lives so they can recover.
- When used as prescribed, buprenorphine significantly lowers the risk of opioid overdose if you take other opioids.
- There are two kinds of buprenorphine. Buprenorphine-naloxone also contains naloxone. Naloxone is not absorbed when the medication is taken as directed (under the tongue). If the medication is injected, however, the naloxone will be absorbed and can cause severe opioid withdrawal.
- There is no limit to how long a person can take buprenorphine. It is recommended that most people be on the medication long-term.
- Side effects may occur and are typically mild and improve over time. These can include constipation, sweating, headache, dizziness, trouble sleeping, nausea, and sleepiness. If these occur, notify your health care provider, nurse, or pharmacist.
- Over time, your body will adjust to buprenorphine. If you stop taking it suddenly, you will feel withdrawal symptoms within a few days.
- A common side effect of buprenorphine and other opioid medications is constipation. To prevent constipation: stay hydrated, eat plenty of fiber, and start taking an over-the-counter stool softener. If you do not have a bowel movement in over 24-48 hours, try an over-the-counter laxative. Talk to the pharmacist to help choose the best one for you.
How to take buprenorphine
- You will receive buprenorphine in either film or tablet form.
- Do not swallow the medicine. It will not work if swallowed.
- Place the medication under your tongue and allow it to fully dissolve. This can take 5-15 minutes.
- Drinking water before taking buprenorphine may help it dissolve faster.
- Do not drink water while the medication is under your tongue.
- Do not eat, drink, talk, or smoke while the medication is dissolving.
- After the medication has dissolved, do not smoke, eat, or drink for at least 15 minutes.
- To prevent tooth decay, rinse your mouth with water 30 minutes after taking the medication.
CAUTION
- Continue taking your regular buprenorphine dose, even if you feel better. Stopping may cause withdrawal and cravings. Your risk of overdose will be much higher if you use opioids without being on this medication.
- Do not start taking or increase your use of other sedative medications like benzodiazepines. The combination of buprenorphine and other sedatives could make you so sleepy that you may stop breathing.
- Do not drink more than your usual amount of alcohol while starting buprenorphine.
- Do not drive when you first start buprenorphine because it may slow your reaction time. Wait until you know how it affects you.
- Remember that changes in opioid use patterns can alter your tolerance and increase your risk of opioid overdose. If you use other opioids, take steps to lower the risk—carry naloxone, never use alone, or call the Never Use Alone lifeline (877-696-1996).
Buprenorphine dose and time taken
________mg @___:____
Overdose Prevention
- Using drugs is risky. If you use drugs, lower your risk of dying from an opioid overdose with the following strategies:
- Naloxone
- Today you received naloxone or a prescription for naloxone. This is an opioid overdose reversal medication. It is safe to use on anyone you suspect is experiencing an opioid overdose.
- Visit stopoverdose.org or talk to your provider, nurse, or pharmacist to learn more.
- Try not to use alone
- If you are not with other people, connect with a confidential service like neverusealone.com. This peer-led service will send someone to help if you stop responding during a chat or phone call.
- Start low & go slow
- You can't know the full contents or strength of drugs. If you use, start with a very small amount to see how it affects you.
- Be extra cautious if you have low tolerance (for example, after not using for awhile). If you decide to use more, slowly increase in small amounts.
- Watch and wait before next person uses
- If you’re with a group of people, take turns to see how the drug is affecting people. Someone needs to be able to ask for help.
- Avoid mixing drugs
- Mixing drugs increases your risk. If you use multiple drugs, try to use one at a time and use less of each.
- Know the signs of opioid overdose and how to respond.
- If someone is unresponsive or has unusual or no breathing, call 911 and give them naloxone and rescue breaths.
- Always have naloxone
- Tell others you have it, where it is, and when to use it.
- Treatment with methadone or buprenorphine
- These medications, if taken as directed, lower the risk of death by over 50%.
- If you need help finding a treatment provider, call the Washington Recovery Helpline at 866-789-1511 or go to warecoveryhelpline.org.
References
- American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Opioids, Hatten BW, Cantrill SV, et al. Clinical Policy: Critical Issues Related to Opioids in Adult Patients Presenting to the Emergency Department. Ann Emerg Med. 2020;76(3):e13-e39. doi:10.1016/j.annemergmed.2020.06.049
- American College of Obstetricians and Gynecologists Committee on Health Care for Underserved Women; American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070-1076. doi:10.1097/AOG.0b013e318256496e
- American College of Obstetricians and Gynecologists. Opioid use and opioid use disorder in pregnancy. ACOG Committee Opinion No. 711. Obstet Gynecol. 2017;130(2):e81-e94.
- Centers for Disease Control and Prevention. Opioid use disorder and pregnancy. Updated April 24, 2024. Accessed August 15, 2025. https://www.cdc.gov/opioid-use-during-pregnancy/treatment/
- Chambers LC, Hallowell BD, Zullo AR, et al. Buprenorphine dose and time to discontinuation among patients with opioid use disorder in the era of fentanyl. JAMA Netw Open. 2023;6(9):e2334540. doi:10.1001/jamanetworkopen.2023.34540
- Cobb J, Craig W, Richard J, et al. A Retrospective Study of Acute Postoperative Pain After Cesarean Delivery in Patients With Opioid Use Disorder Treated With Opioid Agonist Pharmacotherapy. J Addict Med. 2022;16(5):549-556. doi:10.1097/ADM.0000000000000964
- Cohen SM, Straus E, Fiellin DA, et al. Hospital-Based Methadone and Buprenorphine Initiation Practices by Addiction Consult Services. JAMA Netw Open. 2025;8(8):e2526077. Published 2025 Aug 1. doi:10.1001/jamanetworkopen.2025.26077
- Crotty K, Freedman KI, Kampman KM. Executive Summary of the Focused Update of the ASAM National Practice Guideline for the Treatment of Opioid Use Disorder. J Addict Med. 2020;14(2):99-112. doi:10.1097/ADM.0000000000000635
- D'Onofrio G, O'Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474
- Dunn KE, Bird HE, Bergeria CL, Ware OD, Strain EC, Huhn AS. Operational definition of precipitated opioid withdrawal. Front Psychiatry. 2023;14:1141980. Published 2023 Apr 20. doi:10.3389/fpsyt.2023.1141980
- Ecker J, Abuhamad A, Hill W, et al. Substance use disorders in pregnancy: clinical, ethical, and research imperatives of the opioid epidemic: a report of a joint workshop of the Society for Maternal-Fetal Medicine, American College of Obstetricians and Gynecologists, and American Society of Addiction Medicine. Am J Obstet Gynecol. 2019;221(1):B5-B28.
- Englander H, Thakrar AP, Bagley SM, Rolley T, Dong K, Hyshka E. Caring for Hospitalized Adults With Opioid Use Disorder in the Era of Fentanyl: A Review. JAMA Intern Med. 2024;184(6):691-701. doi:10.1001/jamainternmed.2023.7282
- Grande LA, Cundiff D, Greenwald MK, Murray M, Wright TE, Martin SA. Evidence on buprenorphine dose limits: a review. J Addict Med. 2023;17(5):509-516. doi:10.1097/ADM.0000000000001189
- Herring AA, Perrone J, Nelson LS. Managing opioid withdrawal in the emergency department with buprenorphine. Ann Emerg Med. 2019;73(5):481-487. doi:10.1016/j.annemergmed.2018.11.032
- Jones HE, Fischer G, Heil SH, et al. Maternal Opioid Treatment: Human Experimental Research (MOTHER) – approach, issues, and lessons learned. Addiction. 2012;107(suppl 1):28-35. doi:10.1111/j.1360-0443.2012.04036.x
- Khazanchi R, Wachman EM, Schiff DM, Modest A, Saia KA, Hsu HE. Mandatory child protective services reporting for substance-exposed newborns and peripartum outcomes: a difference-in-differences analysis. JAMA Pediatr. Published online May 6, 2024. doi:10.1001/jamapediatrics.2024.0903
- Link HM, Jones H, Miller L, Kaltenbach K, Seligman N. Buprenorphine-naloxone use in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2(3):100179. doi:10.1016/j.ajogmf.2020.100179
- Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. J Addict Med. 2015;9(5):358-367. doi:10.1097/ADM.0000000000000166
- Martin CE, Shadowen C, Thakkar B, et al. Buprenorphine dosing for the treatment of opioid use disorder through pregnancy and postpartum. Curr Treat Options Psychiatry. 2020;7(3):375-399. doi:10.1007/s40501-020-00221-z
- McCarthy JJ, Leamon MH, Finnegan LP, Fassbender C. Opioid dependence and pregnancy: minimizing stress on the fetal brain. Am J Obstet Gynecol. 2017;216(3):226-231. doi:10.1016/j.ajog.2016.10.003
- Min JE, Guerra-Alejos BC, Yan R, et al. Opioid coprescription through risk mitigation guidance and opioid agonist treatment receipt. JAMA Netw Open. 2024;7(5):e2411389. doi:10.1001/jamanetworkopen.2024.11389
- Patrick SW, Barfield WD, Poindexter BB, Committee on Fetus and Newborn, Committee on Substance Use and Prevention. Neonatal opioid withdrawal syndrome. Pediatrics. 2020;146(5):e2020029074. doi:10.1542/peds.2020-029074
- Reindel KL, DeAngelis MJ, Ferrara AS, et al. An exploratory study of Suboxone (buprenorphine/naloxone) film splitting: cutting methods, content uniformity, and stability. Int J Pharm Compd. 2019;23(3):258-263.
- Saia KA, Schiff D, Wachman EM, et al. Caring for pregnant women with opioid use disorder in the USA: expanding and improving treatment. Curr Obstet Gynecol Rep. 2016;5(3):257-263. doi:10.1007/s13669-016-0168-9
- Saxon AJ. Short-acting, full agonist opioids during initiation of opioid agonist treatment in the fentanyl era. JAMA Netw Open. 2024;7(5):e2411398. doi:10.1001/jamanetworkopen.2024.11398
- Young LW, Ounpraseuth ST, Merhar SL, et al; ACT NOW Collaborative. Eat, sleep, console approach or usual care for neonatal opioid withdrawal. N Engl J Med. 2023;388(25):2326-2337. doi:10.1056/NEJMoa2214470
- Washington State Department of Children, Youth, and Families. Plan of safe care: healthcare providers. DCYF website. Accessed August 14, 2025. https://www.dcyf.wa.gov/safety/plan-safe-care/Healthcare-Providers
- Zedler BK, Mann AL, Kim MM, et al. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: a systematic review and meta-analysis of safety in the mother, fetus and child. Addiction. 2016;111(12):2115-2128. doi:10.1111/add.13462


 I’M A CLINICIAN
I’M A CLINICIAN I’M A PATIENT
I’M A PATIENT